
Tyler Irving
February 22, 2016
This story originally appeared on U of T Engineering News.
Professor Hai-Ling Margaret Cheng (IBBME, ECE) was working as an electrical engineer in the aerospace and defence industry when she had an epiphany: she realized the signal-processing techniques she was using to improve radar for remote sensing could also enhance magnetic resonance imaging (MRI) scans. In 1999, she left her job to enroll in a PhD program in medical biophysics. “It was the best decision I ever made, and I’ve loved every day of my job ever since,” she says.
Cheng and her team are dedicated to improving MRI technology. Their techniques could help physicians diagnose cancer in earlier stages, when the chances of successful treatment are highest. They could also enable entirely new fields of practice, such as personalized medicine.
MRI scanners provide detailed, three-dimensional images of the interior of the body without the need for invasive surgery or radiation from x-rays. Though the technology is now well-established in most hospitals, there are always things that can be improved.
One way to enhance MRI scans is to use contrast agents, chemicals that give off a strong MRI signal and can be injected into the body to highlight specific tissues or organs. For example, in cancer treatment doctors often use contrast agents to illuminate small blood vessels. Because tumours grow so quickly, the blood vessels that feed them tend to be poorly formed and leaky, so looking for tiny blood leaks is a good way to find tumours.
Unfortunately, this method only detects cancers that have progressed to a more advanced stage — and often by that point, it’s too late. To catch cancer earlier, researchers must target the cancer cells themselves. A few years ago, Cheng noticed that cancer cells had a particular affinity for manganese, an element often used in contrast agents. “Not only did cancer cells light up more compared to normal cells, but the more aggressive cancer cells lit up more than the less malignant ones,” she says.
The discovery led to a collaboration with Professor Xiao-an Zhang in U of T’s Department of Chemistry to design manganese-based contrast agents that will light up individual cancer cells, rather than the blood vessels around them. This should allow them to detect smaller tumours and remove them before the cancer progresses.
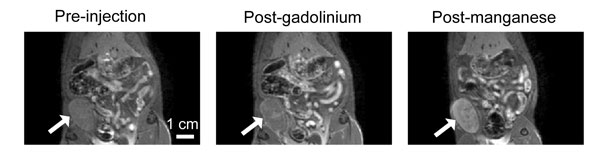
The arrow in these three MRI scans indicates a breast cancer tumour before and after injection with two different contrast agents. The conventional gadolinium-based contrast agent (middle image) barely changes the visibility of the tumour, but the new manganese-based one (right image) makes the tumour much brighter and easier to see. (Image credit: Hai-Ling Margaret Cheng)
Being able to use MRI to track individual cells holds promise for another application: personalized medicine. Over the past decade, researchers have become increasingly adept at working with stem cells, which are capable of developing into any other type of cell, from muscle to nerves to blood. Eventually, the goal is to implant stem cells or lab-grown tissues and organs into the body to repair damage from disease or traumatic accidents.
But if replacement cells are to be implanted into humans, doctors will need to track how they’re growing over the course of several weeks. Cheng believes MRI can help. In a recent project, her students labelled embryonic stem cells with a new MRI contrast agent and then watched as they turned into beating heart cells in a dish. “We demonstrated that the labelling procedure doesn’t affect their ability to turn into normal, functional heart cells,” says Cheng. If this works in the body as well, it could allow researchers to follow implanted cells in unprecedented detail as they integrate into their new environment.
For Cheng, the promise of changing lives through earlier cancer detection or new abilities to repair damaged organs provides a powerful motivation. “What keeps me going through setbacks and failed experiments is that I know we are going to be making a real difference,” she says. “My children will have access to tests and treatments that we just don’t have today. I love what I do, and I’m very lucky to be able to say that.”
More information:
Leanne Moss
Communications Assistant
The Edward S. Rogers Sr. Department of Electrical & Computer Engineering
416-978-1999; leanne.moss@utoronto.ca